X
Bio Conjugation Price And Quantity
- 150.00 - 300.00 INR
- 150 INR
- 50 Gram
Bio Conjugation Trade Information
- Per Day
- Days
- cartons & wooden packing
- All India
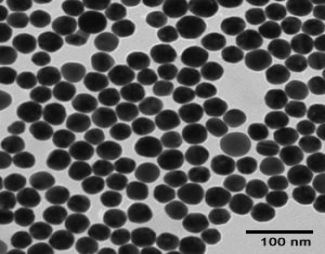
Product Description
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Bio Pure Nano' category
 |
ULTRANANOTECH PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |